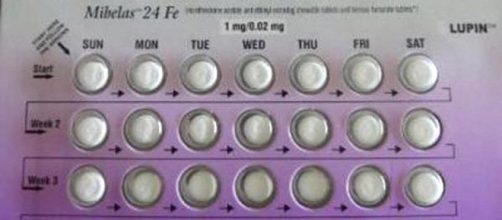
Birth Control tablets have been recalled by the Food and Drug Administration (FDA), according to CBS News. The recall is on the Mibelas 24 Fe birth control tablets that are distributed nationwide by Lupin Pharmaceuticals. The FDA explained the reason for the recall.
Packaging error
Actually, there is nothing wrong with the tablets. The error is in the package they are in. For example, the package doesn't rotate to display the right pill for the day it is supposed to be taken. Because of the faulty rotation, the tablets are out of sequence. Besides, the lot number and expiration date on the package cannot be seen.
Those who take the tablets on a regular basis might have noticed the faulty packaging and have already alerted their doctor. On the other hand, there might be users of Mibelas 24 Fe birth control tablets who have not notice any differences at all.
Tablets out of order
Four placebo tablets were placed incorrectly at the beginning of the sequence. That makes the active tablets out of their proper place in the rotation. The FDA says this could cause unwanted pregnancies in women who took the tablets on the wrong days.
No one has reported having taken the tablet on a wrong day or if there have been any adverse effects. Of course, it is too soon to tell if there is an unwanted pregnancy.
Lupin Pharmaceuticals
Lupin Pharmaceuticals is among the top five pharmaceutical companies in India, but Baltimore, Maryland is the headquarters for sales and marketing. Healthcare providers and patients around the world have been trusting the company for its medications. However, this recall seems to be one of its kind.
Lupin Pharmaceuticals is in the process of notifying all their distributors and customers that there is a nationwide recall on the popular brand of birth control tablets that women take to keep from getting pregnant.
Women should take immediate action if they believe they have been affected because of the packaging error. They should notify their doctor or healthcare provider. Also, they can return the tablets to the place where they purchased them to get a full refund.
To put consumers at ease about the recall, more information is listed on the FDA's website.
If after reading the information and they still have questions about the tablets and packaging, they should call Lupin Pharmaceuticals at 1-800-399-2561 during regular business hours.
Hopefully, women find out about this recall before they take any of the birth control tablets.